Metabolic and innate immune cues merge into a specific inflammatory response via unfolded protein response (UPR)
Résumé
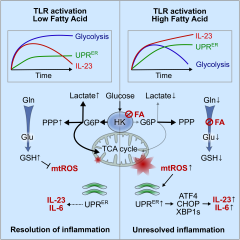
Innate immune responses are intricately linked with intracellular metabolism of myeloid cells. Toll-like
receptor (TLR) stimulation shifts intracellular metabolism toward glycolysis, while anti-inflammatory
signals depend on enhanced mitochondrial respiration. How exogenous metabolic signals affect the
immune response is unknown. We demonstrate that TLR-dependent responses of dendritic cells (DC)
are exacerbated by a high fatty acid (FA) metabolic environment. FA suppress the TLR-induced
hexokinase activity and perturb tricarboxylic acid cycle metabolism. These metabolic changes
enhance mitochondrial reactive oxygen species (mtROS) production and, in turn, the unfolded protein
response (UPR) leading to a distinct transcriptomic signature, with IL-23 as hallmark. Interestingly,
chemical or genetic suppression of glycolysis was sufficient to induce this specific immune response.
Conversely, reducing mtROS production or DC-specific deficiency in XBP1 attenuated IL-23
expression and skin inflammation in an IL-23-dependent model of psoriasis. Thus, fine-tuning of innate
immunity depends on optimization of metabolic demands and minimization of mtROS-induced UPR.
Fichier principal
 CELL-D-18-00957R2 -Manuscript-RevisedFinal.pdf (10.8 Mo)
Télécharger le fichier
CELL-D-18-00957R2 -Supplementary-RevisedFinal-1.pdf (4.51 Mo)
Télécharger le fichier
CELL-D-18-00957R2 -Manuscript-RevisedFinal.pdf (10.8 Mo)
Télécharger le fichier
CELL-D-18-00957R2 -Supplementary-RevisedFinal-1.pdf (4.51 Mo)
Télécharger le fichier
| Origine | Fichiers produits par l'(les) auteur(s) |
|---|
Loading...