Cathepsin D is partly endocytosed by the LRP1 receptor and inhibits LRP1-regulated intramembrane proteolysis.
Résumé
The aspartic protease cathepsin-D (cath-D) is a marker of poor prognosis in breast cancer that is overexpressed and hypersecreted by human breast cancer cells. Secreted pro-cath-D binds to the extracellular domain of the β-chain of the LDL receptor-related protein-1 (LRP1) in fibroblasts. The LRP1 receptor has an 85-kDa transmembrane β-chain and a noncovalently attached 515-kDa extracellular α-chain. LRP1 acts by (1) internalizing many ligands via its α-chain, (2) activating signaling pathways by phosphorylating the LRP1β-chain tyrosine and (3) modulating gene transcription by regulated intramembrane proteolysis (RIP) of its β-chain. LRP1 RIP involves two cleavages: the first liberates the LRP1 ectodomain to give a membrane-associated form, LRP1β-CTF, and the second generates the LRP1β-intracellular domain, LRP1β-ICD, that modulates gene transcription. Here, we investigated the endocytosis of pro-cath-D by LRP1 and the effect of pro-cath-D/LRP1β interaction on LRP1β tyrosine phosphorylation and/or LRP1β RIP. Our results indicate that pro-cath-D was partially endocytosed by LRP1 in fibroblasts. However, pro-cath-D and ectopic cath-D did not stimulate phosphorylation of the LRP1β-chain tyrosine. Interestingly, ectopic cath-D and its catalytically inactive (D231N)cath-D, and pro-(D231N)cath-D all significantly inhibited LRP1 RIP by preventing LRP1β-CTF production. Thus, cath-D inhibits LRP1 RIP independently of its catalytic activity by blocking the first cleavage. As cath-D triggers fibroblast outgrowth by LRP1, we propose that cath-D modulates the growth of fibroblasts by inhibiting LRP1 RIP in the breast tumor microenvironment.
Fichier principal
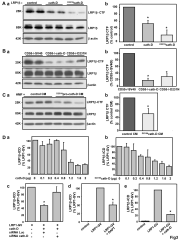 Figure3_13_RIP-3.pdf (414.47 Ko)
Télécharger le fichier
Figure1_13_RIP-1.pdf (327.91 Ko)
Télécharger le fichier
Figure2_13_RIP-2.pdf (296.44 Ko)
Télécharger le fichier
Figure4_13_RIP-4.pdf (298.88 Ko)
Télécharger le fichier
inserm-00646373_edited.pdf (559.66 Ko)
Télécharger le fichier
paper_LRP1_RIP22.pdf (329.53 Ko)
Télécharger le fichier
Figure3_13_RIP-3.pdf (414.47 Ko)
Télécharger le fichier
Figure1_13_RIP-1.pdf (327.91 Ko)
Télécharger le fichier
Figure2_13_RIP-2.pdf (296.44 Ko)
Télécharger le fichier
Figure4_13_RIP-4.pdf (298.88 Ko)
Télécharger le fichier
inserm-00646373_edited.pdf (559.66 Ko)
Télécharger le fichier
paper_LRP1_RIP22.pdf (329.53 Ko)
Télécharger le fichier
| Origine | Fichiers produits par l'(les) auteur(s) |
|---|
| Origine | Fichiers produits par l'(les) auteur(s) |
|---|
| Origine | Fichiers produits par l'(les) auteur(s) |
|---|
| Origine | Fichiers produits par l'(les) auteur(s) |
|---|
| Origine | Fichiers produits par l'(les) auteur(s) |
|---|
| Origine | Fichiers produits par l'(les) auteur(s) |
|---|